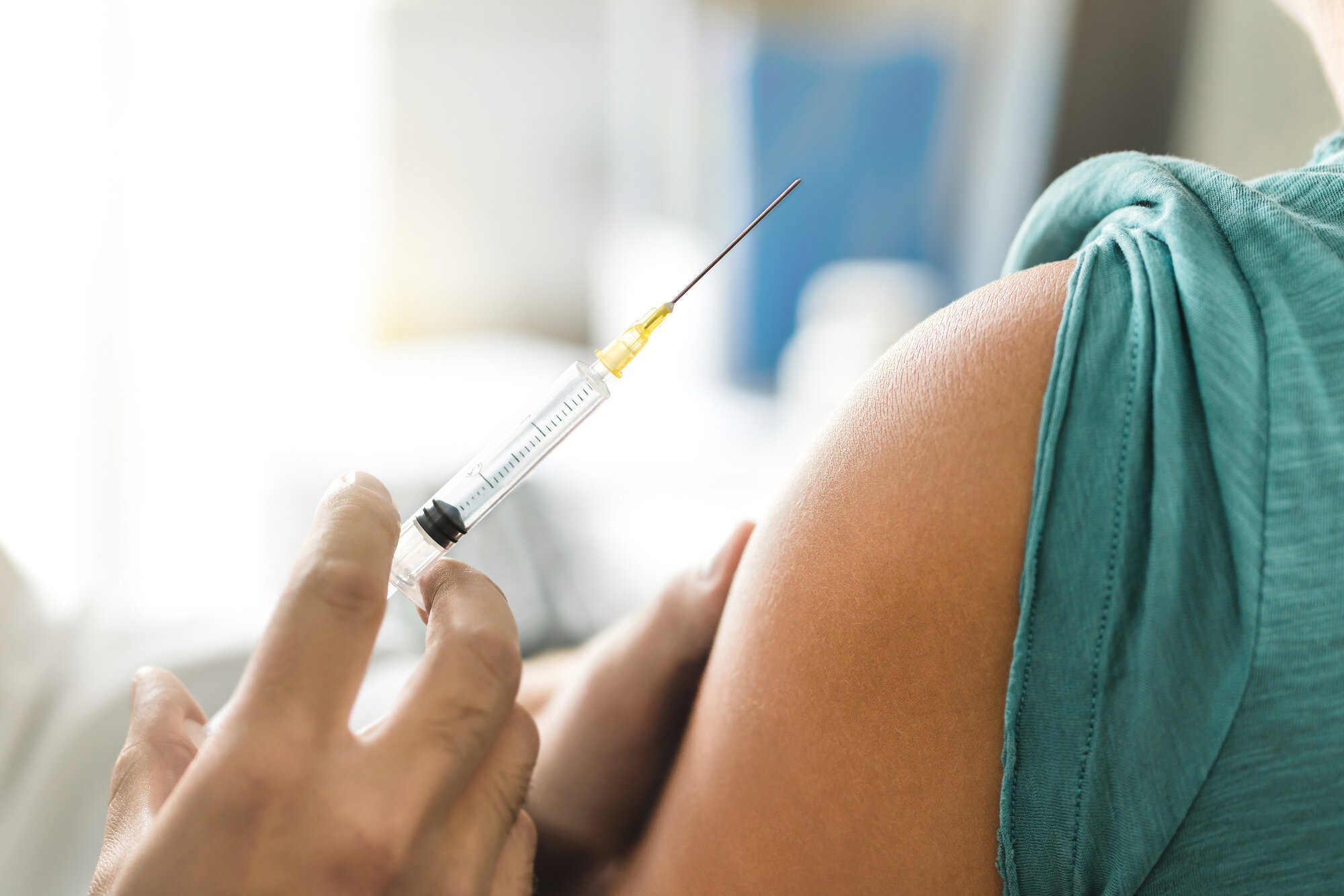
- Two coronavirus vaccine candidates stopped working in medical trials, indirectly showing that the vaccine development process works.
- The measures put in place will guarantee that just the drugs that are both reliable and safe will be authorized.
- University of Queensland and CSL consented to stop medical trials in Australia after scientists discovered the drug had an unexpected negative effects: false-positive HIV diagnoses. The drug provided a robust action against COVID-19, but a repair might take up to 12 months.
- The Sanofi/GSK drug stopped working to elicit an efficient immune action in individuals older than49 The 2 companies will begin a brand-new trial in February, and their brand-new formula might be ready by the end of 2021.
Modern innovation enabled numerous teams to begin coronavirus vaccine screening in the early months of the pandemic. The early release of the SARS-CoV-2 genome was the very first breakthrough that made it possible to start screening in laboratories immediately. This led to Stage 1 clinical trials by April and Phase 3 conclusions by mid-November for the 3 frontrunners. While volunteers got the speculative drugs or a placebo, the business involved in these jobs started setting production and logistics in motion so that the vaccine could be dispersed to those most in need as soon as regulators like the FDA issued emergency situation approvals.
The Pizer/BioNTech drug was approved in the UK and is expected to get a similar approval in America and the European Union. Moderna’s prospect need to follow. The more controversial AstraZeneca/Oxford drug could likewise be approved in the coming months, although the United States may require another Stage 3 trial for it. That’s the short story of the COVID-19 vaccine advancement history to date in Western countries. Contribute to that the Russian vaccine that was approved in August and the a number of Chinese vaccines that are already utilized in the wild ahead of the Phase 3 research study conclusion, and you get the big COVID-19 vaccine photo for 2020.
However, as all of that was taking place, more and more individuals grew distressed at the idea of getting a vaccine that was developed in record time. Fake news campaigns on social media, as well as the politicization of vaccines ahead of the US presidential election, even more eroded public trust in vaccines
Reports on Friday said that not one however 2 various coronavirus vaccine candidates that were in different phases of human trials failed. That may appear like problem that can affect stock appraisal and force federal governments who were banking on those drugs to look for additional services. But, oddly enough, this is also very good news for the whole coronavirus vaccine research and development procedure. The failure of essential drugs proves to the world the research openness that governments and pharmaceutical business pledged. It likewise proves there’s extreme analysis in this specific field which science is the only thing that will matter when it concerns authorizing vaccines for public use. Those drugs and vaccines that are authorized will not simply work in COVID-19 therapy; they’ll likewise be safe for the public.
The Australian government has actually simply canceled a regional coronavirus vaccine development program, as the trials showed that the vaccine interfered with HIV diagnosis. That’s obviously not the kind of reaction anyone would desire from the vaccine.
Developed by the University of Queensland (UQ) and biotech company CSL, the vaccine prospect elicited a “robust” immune action without severe negative impacts in a Stage 1 trial. But the UQ/CSL needed to be stopped, as the drug requires to be reengineered. The process may use up to 12 months, per Reuters
There’s still wish for the UQ/CSL project to move forward, however the primary focus will be making a vaccine that can be utilized today. CSL could make extra dosages of the Oxford vaccine to make up for the failure.
Like other Western federal governments, the Australian leadership tattooed deals with different vaccine makers, consisting of Pfizer and Novavax. Many vaccines will need two shots taken a few weeks apart.
A various Reuters report notes the second major vaccine failure of the week. Sanofi and GSK have revealed they’re required to delay their vaccine prospect, as the drug showed an insufficient immune reaction in older people. This group is most at risk of serious COVID-19 and death. Over the summertime, we’ve seen health professionals voicing their concerns about vaccine effectiveness in the older population and those overweight or overweight. Vaccines from Pfizer and Moderna have revealed good effectiveness throughout age.
The Sanofi/GSK drug elicited “an immune response comparable to patients who recovered from COVID-19 in adults aged 18 to 49 years, however a low immune response in older adults likely due to an inadequate concentration of the antigen” after Stage 1/2 trials.
Like with the UQ/CSL vaccine, the Sanofi/GSK vaccine job isn’t dead. If effective, the trial could yield another reliable vaccine by the end of2021
” The research study will include a proposed comparison with an authorized COVID-19 vaccine,” according to Sanofi
The Sanofi vaccine uses the exact same recombinant protein-based tech as a Sanofi flu vaccine. GSK makes the adjuvant, which serves as a booster for the vaccine. The companies planned to manufacture as much as one billion dosages in2021 GSK will continue to make the adjuvants, with some of them anticipated to be provided to other partners.
While everyone was confident about vaccines throughout the year, there was never any warranty these drugs would work. We were constantly facing the possibility that numerous vaccine candidates might stop working– maybe all of them. These negative outcomes should even more increase confidence in the scientific procedure behind vaccine development and persuade more people that the vaccines that are authorized for emergency situation usage are not risky political tricks.
Chris Smith began writing about gadgets as a hobby, and prior to he knew it he was sharing his views on tech things with readers around the world. Whenever he’s not writing about devices he badly fails to keep away from them, although he frantically attempts. But that’s not always a bad thing.
No comments:
Post a Comment